|
|


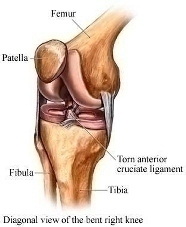
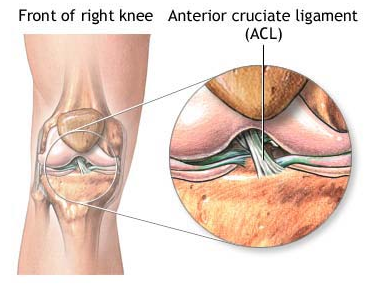
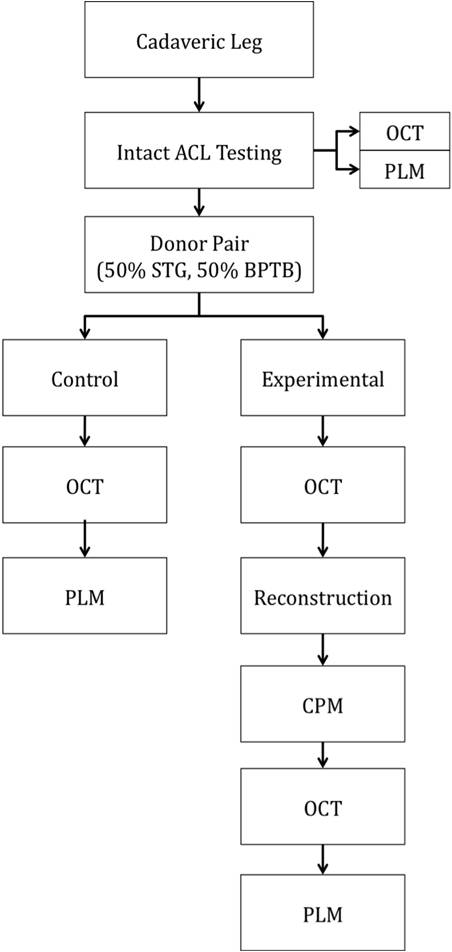
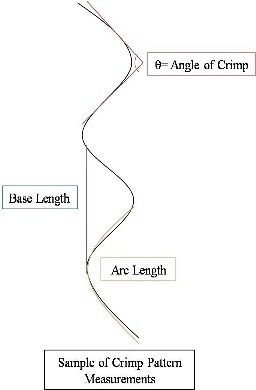
Abstract Anterior cruciate ligament (ACL) graft reconstruction relies on the effectiveness of graft materials and surgical procedures to successfully restore knee function. Current materials and methods fall short of providing adequate recovery and commonly result in a loss of tension (laxity) independent of the type of graft used. Past research has shown differences in graft performance based on graft origin: ACL donor, bone-patella-tendon-bone (BPTB), hamstring (STG). Further research has demonstrated a correlation between crimping patterns in the collagen fibrils of ligaments and the ability of the graft to withstand tension. No studies thus far have combined morphological differences between grafts (BPTB, STG, ACL) with their varying performance in tensile tests under continuous passive knee motion (CPM). This study will attempt to explain that relationship. BPTB, STG and ACL grafts from five pairs of cadaveric legs will be imaged using noninvasive optical coherence tomography (OCT). One leg from each pair will serve as a control, and the relevant tissues will be imaged using polarized light microscopy (PLM). Each control’s corresponding leg will then undergo CPM testing. The BPTB, STG and ACL grafts will all be individually tested and monitored for tension loss using a materials testing system (MTS) machine. After CPM, each graft will be reimaged with OCT as well as PLM. Results from before and after OCT imaging, as well as morphological differences between the control and experimental specimens, as seen through PLM, will be compared to tension performance from CPM. Marked differences in tension loss should stem from morphological differences between graft types. Through this research, we hope to enable better predictions to be made regarding the extent of joint laxity displayed by specific grafts post ACL reconstruction surgery. Experiment A. Preparation of Specimen Grafts will be harvested from five pairs of cadaveric knees simultaneously and then frozen at -20°C until needed. For this study, a test specimen is defined as a pair of legs belonging to the same body. Because PLM compromises tissue integrity, it cannot be performed before mechanical testing. Since tissues from each leg of the same body should be morphologically similar, a randomly selected knee will serve as a control and only be used for preliminary imaging analysis, while the other will undergo ACL reconstruction, mechanical testing, and post-testing imaging analysis, as shown in Appendix A. A surgeon will harvest the STG, BPTB and ACL grafts from each leg and perform three separate ACL reconstructions on each leg with each graft. Before experimental testing, the legs will be thawed for 24 hours, after which the skin and soft tissues of the leg will be removed. Osteotomies on the femur and tibia will then be performed 15cm proximal and distal to the medial joint line, respectively. Capsuloligamentous structures will be kept to enhance knee stability and allow for more accurate knee motion (Arnold, et al., 2005). For harvesting the STG graft, 10-11cm portions of the tendons will be removed, stripped of adherent muscle fibers, and trimmed to equal lengths (Figure 1A). These portions will be doubled over and sutured together to form a STG graft, as seen in Figure 1B, with a diameter of approximately 7-9mm (L. Pinczewski, et al., 1998; Simonian, Cole, & Bach, 2006; S. L. Y. Woo et al., 2000). The middle one-third of the patellar tendon, approximately 8-10mm wide, will be extracted along with bone plugs from the patella and tibia to form the BPTB graft (Figure 2). Bone plugs should have the same width as the tendon grafts, ranging anywhere from 9-25mm long (L. Pinczewski, et al., 1998; Simonian, et al., 2006; S. L. Y. Woo, et al., 2000). B. Pre-CPM Imaging Analysis PLM and OCT will be performed on the control grafts. Graft tissues to be mechanically stressed will also undergo OCT because this imaging technique is noninvasive and nondestructive. Polarized Light Microscopy: The procedure for analysis of ligament and tendon tissue by PLM will be performed as directed by Franchi et al. (2008). The specimens will be prepared in a 10% neutral buffered formalin solution for 24 hours to initiate fixation. Before solidifying the specimens in paraffin, they will be dehydrated with alcohol. Then, 10mm cross-sections will be stained with 5% Picrosirius Red and digitally imaged . The collected images will be processed using ImageJ (NIH). Three tissue samples will be obtained from each graft and ten random images will be taken per tissue. The software will return values for the number of crimps in the sample, the angle of the crimp pattern sinusoid, and the base- and arc-lengths of each crimp pattern. Optical Coherence Tomography: The procedure for OCT will be performed as directed by Dr. Yu Chen at the Fischell Department of Bioengineering, University of Maryland, College Park (2009). In order to obtain the best 3-D image with improved speed and sensitivity, swept source/Fourier domain (SS-FD) OCT imaging will be performed with a buffered Fourier domain mode- locking (FDML) laser. The laser scans 100nm widths at 1300nm wavelengths, thus generating an axial resolution of 10mm, or 2.3mm in tissue (Yuan, 2009). The laser sweeps over the tissue at a rate of 16kHz with an average output power of 12mW. Both the lateral resolutions and magnifications of images taken can be altered to create optimal images during experimentation. The graft tissue will be placed on an imaging platform where an infrared FD laser will scan the sample. The penetrating beam will reflect only a small amount of light back to collecting lens (Yuan, 2009).. OCT will thus reflect light off the crest and trough of each crimp in the tissue sample; intervening dark bands represent the slope of the crimp. The period length of each crimp will be the width of a band pair. C. Graft Pretensioning Protocol All grafts should be uniformly pretensioned with 20-80N of force within a range of 0-25º of knee flexion for 20 minutes. Though suggestions for amounts of tensioning vary, the consensus among surgeons is that pretensioning is necessary (Blythe, et al., 2006). Since this study aims to mimic typical ACL reconstruction as closely as possible, all grafts will be pretensioned prior to insertion (Arnold, et al., 2005; Dargel, et al., 2007). D. Biomechanical Testing - Continuous Passive Knee Motion This study chooses to replicate CPM as opposed to cyclical loading. The latter is intended to model normal walking, but a typical patient uses crutches for a period of at least three months. In addition, many patients use a CPM machine during the initial post-reconstruction period. After graft harvest, a graft will be mounted onto an MTS machine to replicate CPM. In order to avoid causing tissue damage to the grafts, cryoclamps will be utilized to achieve a secure and effect clamping on the MTS machine. The cryoclamps used will be TestResources G227 Stainless Steel Corrugated Clamps, and will be fitted into the MTS. The test will be modeled after the OptiFlex® 3 Knee CPM device commonly used for post-reconstruction rehabilitation ("OptiFlex 3 Knee Continuous Passive Motion," 2009). For this study, the average patient’s CPM device settings post-reconstruction will be a force of 200N. The knee will undergo 1500 cycles at a rate of .5 cycles per second ("OptiFlex 3 Knee Continuous Passive Motion," 2009). While the grafts undergo CPM testing, the MTS will transmit and record real-time data of graft tension and the grafts will be continuously sprayed with saline solution to more accurately reproduce physiological conditions and prevent dehydration. |
Our Research |
E. Post-CPM Imaging Analysis After CPM, the graft will be removed from the knee and will undergo PLM and OCT imaging, as described in part B. F. Statistical Analysis The MTS will measure loss in tension as a function of the number of cycles. To compare performance among grafts, an independent two-sample t-test will be applied, assuming an equal sample size (n=number of cycles) with equal variance. The normality of the data distribution will be confirmed with the Shapiro Wilk Test (Ahmed & McLean, 2002). The Wilcoxon Signed Rank Test will be used in place of the t-test in case the data is not normally distributed. The Pearson correlation coefficient will be calculated to determine the dependence of tension loss on the number of cycles; the coefficient for each graft will be compared to determine which graft was most affected by CPM (Ahmed & McLean, 2002). After tissue imaging, an ANOVA test will be used to assess differences among grafts between the before- and after-images of the same graft (Franchi, et al., 2009). |
For questions or comments, contact web liaison |


