|
|
|
The Problem: |
|
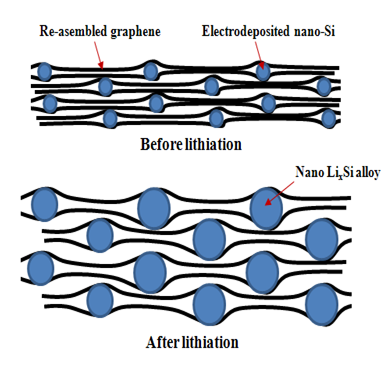
Silicon is an ideal material for a lithium-ion anode because it has an increased capacity, a high energy density, and a lower cost in comparison with other anode materials. However, silicon has a tendency to expand by about 400 percent during charge/recharge cycles, which can result in the destruction of the silicon particles. This subsequently causes silicon anode-based batteries to have lower cycle lives, as silicon particles are quickly pulverized and detached from the batteries' internal current collectors. Despite silicon's remarkable advantages, these physical properties have impeded its adoption as an alternative to existing anode materials. |
|
Objective: |
|
Our objective is to fabricate a silicon-graphene composite anode to be used in a rechargeable lithium-ion battery. We believe that the rigid structure of the graphene will hold the silicon nanoparticles firmly in place, thus preventing their pulverization and detachment. This composite should enable the battery to maintain the improved performance properties characterized by silicon anodes without the significant downfalls that normally accompany their use. |
 |
| [Home] [About Us] [Our Research] [The How?] [Timeline] [Links] |
|
|